EIZO Receives FDA 510(k) Clearance for Breast Tomosynthesis for RadiForce RX850 Multi-Modality Monitor
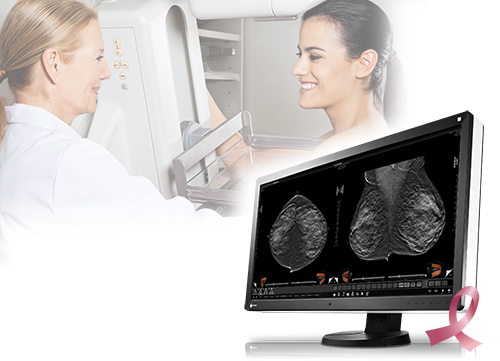 Cypress, CA, November 25, 2015 – EIZO Inc. announced that it has received FDA 510(k) clearance for breast tomosynthesis from the U.S. Food and Drug Administration for its 8 megapixel multi-modality monitor, the RadiForce RX850.
Cypress, CA, November 25, 2015 – EIZO Inc. announced that it has received FDA 510(k) clearance for breast tomosynthesis from the U.S. Food and Drug Administration for its 8 megapixel multi-modality monitor, the RadiForce RX850.
Digital breast tomosynthesis is a method of breast screening that allows the radiologist to view detailed 3-dimensional images of the breast assembled from multiple X-ray pictures. When used in combination with digital mammography, tomosynthesis provides radiologists with multiple screening techniques that can be utilized together for increased diagnostic precision in breast cancer detection. With FDA 510(k) clearance for the RadiForce RX850 in tomosynthesis, mammography, and general radiography, EIZO continues to provide a high degree of customer assurance to medical breast screening professionals and their patients.
The RadiForce RX850’s super high-resolution screen (4096 x 2160) displays 8 megapixels of information with a pixel pitch of 0.1704 mm for viewing medical breast images in exceptional detail. To detect the smallest structures, the monitor offers a high contrast ratio of 1450:1. The deeper black levels distinguish similar shades of gray for sharper monochrome image reproduction.
Product Information
EIZO will exhibit the RadiForce RX850 at the 2015 Radiological Society of North America (RSNA 2015) in Chicago, Illinois from November 29 - December 3. Visit EIZO booth #1714 to see the product. Request a Demo/Meeting
About EIZO
EIZO (TSE:6737), which means image in Japanese, is a visual technology company that develops and manufactures high-end display solutions. EIZO integrates hardware and software technologies with consulting, web hosting, and other services to help customers in business, graphics, gaming, medicine, maritime, air traffic control, and other fields work more comfortable, efficiently, and creatively. Headquartered in Hakusan, Japan, EIZO has R&D and manufacturing facilities in Japan, China, Germany, and the US, and representation in more than 80 countries.
For more information, please contact:
EIZO Inc
5710 Warland Drive
Cypress, CA 90630
USA
Phone: (800) 800-5202
![]() EIZO supports the pink ribbon campaign for early detection of breast cancer.
EIZO supports the pink ribbon campaign for early detection of breast cancer.
All product names are trademarks or registered trademarks of their respective companies. EIZO and RadiForce are registered trademarks of EIZO Corporation.




